Abstract The exploration of hard and superhard materials has always been an important research direction in the field of condensed matter physics, and it also has great application prospects in practical industrial production. Traditional superhard materials such as diamond, cubic boron nitride, etc., are usually made of light elements (BCNO)...
The exploration of hard and superhard materials has always been an important research direction in the field of condensed matter physics, and it also has great application prospects in practical industrial production. Conventional superhard materials such as diamond, cubic boron nitride, etc., are usually composed of light elements (BCNO) in the form of covalent bonds. This strong BCNO covalent bond has good directionality and is resistant to isotropic compression. It is also resistant to shear in different directions and thus exhibits extremely high mechanical strength. However, the defects of traditional superhard materials are also very prominent: diamonds are prone to graphitization, and the synthesis of cubic boron nitride requires unusually harsh temperature and pressure conditions. In addition, pure covalent bonding forms often lead to their electrical extinction or broadband semiconductors, while industrial applications require materials with super-hard mechanical properties and good electrical properties (such as superhard) under many conditions. Coating, wire cutting, press hammer, etc.). Therefore, the search for ultra-hard low-resistance and even metallic materials has become an important research hotspot in recent years. Recently, the Institute of Physics of the Chinese Academy of Sciences/Beijing National Laboratory of Condensed Matter Physics (Financing) Extreme Condition Laboratory EX6 Group Yu Xiaohui Associate Researcher and Surface Physics Laboratory SF10 Group Li Hui Associate Researcher, EX5 Group Yan Changqing Researcher, Jilin University Professor Zhu Pinwen, Professor Zhang Ruifeng from Beijing University of Aeronautics and Astronautics has made important progress in exploring the “metal-electric diamondâ€-ZrB12. They first successfully prepared pure phase ZrB12 samples by means of high temperature and high pressure, and refined the crystal structure by the crystal diffraction spectrum.

Figure 1: Refined ZrB12 crystal structure
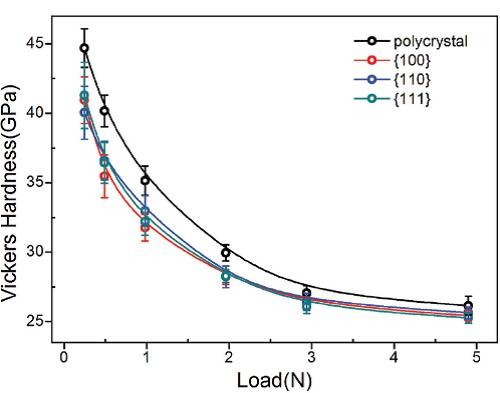
Figure 2: The Vickers hardness of ZrB12 polycrystals and single crystals varies with loading force. Under small loading (25g, 50g), the hardness value exceeds 40 GPa, which is the standard for superhard materials.
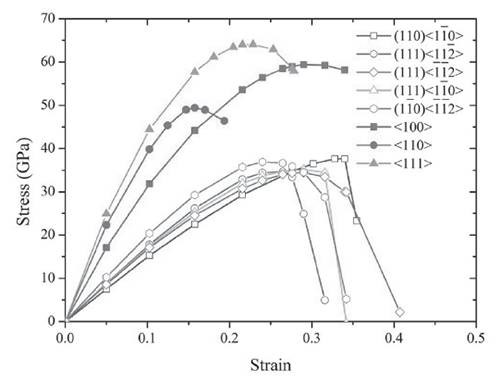
Figure 3: The theoretical mechanical strength of ZrB12 is as high as 34.5 GPa, which is close to the traditional superhard material B6O.
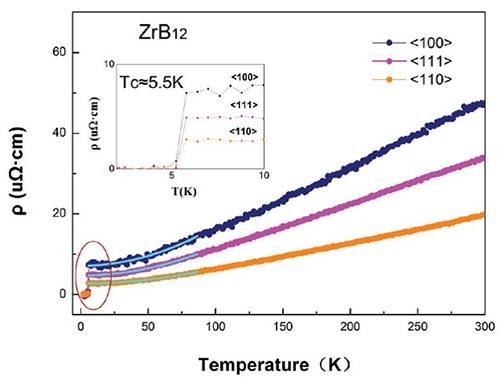
Figure 4: ZrB12 resistivity changes with temperature and exhibits excellent metallability. At room temperature, the resistivity is only 18 μΩ·cm, which is almost equivalent to the metal Pt.
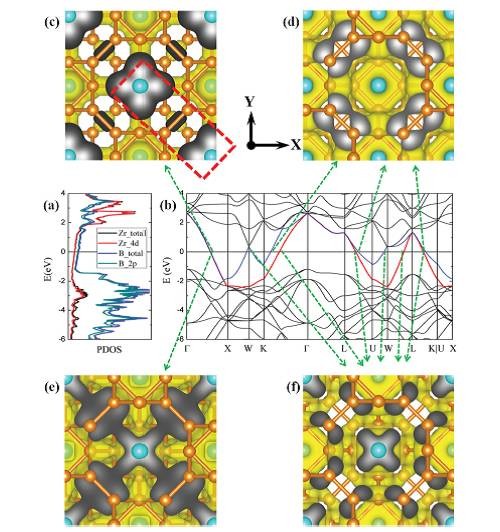
Figure 5: ZrB12 band structure analysis: (a) PDOS; (b) band structure near the Fermi surface; (c)-(f) energy band analysis across the Fermi surface.
Vde Screwdriver,Vde 7Pcs Precision Screwdriver Set,Vde 50Pcs Exchange Screwdriver Set,Vde Pozi Screwdriver
Shanghai Countool In't Trading Co.,ltd , https://www.countool.com
