(Image source: greencarcongress)
According to foreign media reports, researchers at the University of Oregon have successfully improved the catalytic hydrolysis performance of bipolar membranes. This research provides a roadmap for building an electrochemical device, which can benefit from the key feature of the bipolar membrane, which is to generate protons and hydroxide ions inside the device and supply these ions directly to the electrode to produce the final chemical product.
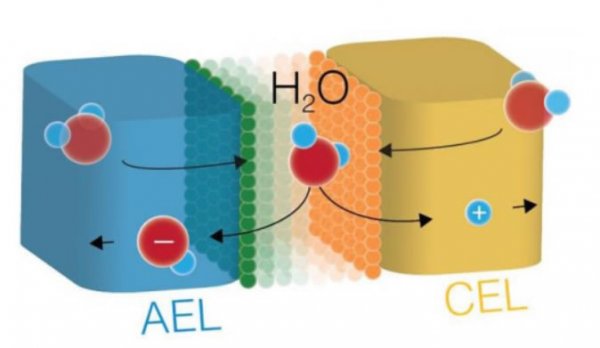
Researcher Oener et al. stated that in a bipolar membrane (BPM), the copolymerized cation exchange layer (CEL) is in contact with the anion exchange layer (AEL). Among them, the CEL layer is composed of fixed anionic groups and movable cations, while the AEL layer is composed of fixed cationic groups and movable anions. When sufficient bias is applied to the BPM, the insoluble hydrolysis will occur at the AEL/CEL interface, that is, H2O→H+ + OH-, then, H+ passes through the CEL layer, and OH- passes through the AEL
Bipolar membrane technology appeared in the 1950s, that is, a layer of ion-exchange polymer was sandwiched with a hydrolytic catalyst layer. This technology has been applied on a small scale in the industrial field, but due to the need to operate at a low current density, its further development is limited.
Shannon W. Boettcher, professor of the Department of Chemistry and Biochemistry at the University of Oregon and founding director of the Oregon Electrochemical Center, said that these applications can use water and electricity to produce hydrogen, capture carbon dioxide from seawater, or directly use carbon dioxide to produce carbon-based fuels. .
Lead researcher Sebastian Z. Oener said that, generally speaking, in the entire system, water-based electrochemical devices such as batteries, fuel cells, and electrolytic cells operate at a single pH value. The system is either acidic or alkaline. "Therefore, it is often necessary to use expensive precious metals to catalyze electrode reactions, such as iridium, one of the rarest metals on earth; or to sacrifice catalyst activity to increase the energy input required by the electrochemical reactor. The bipolar membrane can be at an ideal pH In the environment, each electrocatalyst is locally operated to overcome these problems."
The research team used a membrane electrode assembly to compress the polymer bipolar membrane between two strong porous electrodes. In this way, a large number of bipolar membranes with different hydrolytic catalyst layers can be fabricated, and the activity of each layer can be accurately measured.
The research team found that the exact location of each catalyst layer at the junction of the bipolar membrane, that is, the interface between the hydroxide conductive layer and the proton conductive layer in the bipolar membrane, significantly affects the activity of the catalyst. On this basis, they used a catalyst bilayer membrane to create a bipolar membrane with excellent performance. This bipolar membrane can basically separate water without causing additional energy loss. Researcher Shannon Boettcher said: "The most surprising thing is that it can significantly improve its performance by superimposing different types of catalyst layers. This is simple, but it has not been fully explored."
Oener said that the second key finding is that the hydrolysis reaction occurring in the bipolar membrane is fundamentally related to the reaction occurring on the surface of the electrocatalyst. For example, when hydrogen fuel is produced under basic pH conditions, it can be directly extracted from the water molecules. Extract protons. "This is unique. It is impossible to separate the various steps that occurred in the previous electrochemical reaction. They are related to each other, including electrons and intermediate products, and are carried out continuously. Through the bipolar membrane structure, we can separately perform the chemical step of hydrolytic separation, and Conduct a separate study."
He said the discovery may also improve electrocatalysts for reactions that directly produce reduced fuels from water, such as the use of carbon dioxide waste gas to produce hydrogen or liquid fuels.
Boettcher said that these findings provide experimental mechanical models that can open up the field and stimulate more research. Currently, researchers are applying for a patent for the bipolar membrane technology they developed.
Urine Bag,Urine Collector,Medical Urine Bag,Urine Collection Bag
Henan Diyi Medical Technology Development Co.,Ltd. , https://www.diyimedical.com
